Abstract
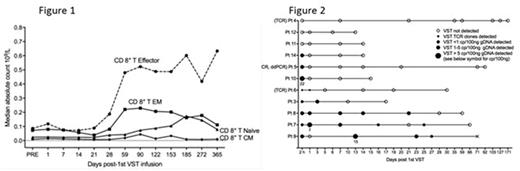
Partially HLA matched 3rd party virus-specific T-cells (VST) from cryopreserved cell banks have been reported to control EBV, CMV and adenovirus specific reactivations after allogeneic stem cell transplantation (HSCT). The duration of viral control and its mechanism are uncertain. We treated 30 HSCT patients who had persistent or recurrent CMV (n=28), EBV (n=1) or adenovirus (n=1) after 2 weeks of ganciclovir or foscarnet (or appropriate therapy for EBV and adenovirus) with infusions of partially HLA-matched, 3rd-party ex vivo expanded VST (median 2 of 6 HLA matches between product and recipient). A total of 50 infusions were administered at a median of 75 days post-HSCT (range 37-349). 17 patients received a single infusion of 3rd party T-cells. We confirmed the results of Leen et al (2013), our data showing an overall response rate of 93% and a virological CR rate of 76% without infusion toxicity or enhanced GVHD. Viral control was observed equally in patients with and without CMV UL54 and UL97 mutations. We followed patients for 12 months to examine durability of viral control, persistence of infused cells and immune reconstitution. Prior to the first T cell infusion, a mean of 39 (range 14-113) days of anti-CMV pharmacotherapy had been administered. Following the final T-cell infusion a mean of 15 (range 0-91) days of anti-CMV pharmacotherapy was given. No patient lost viral control more than 100 days after final infusion. Antiviral therapy was reintroduced in only 5 patients after it had been ceased following T-cell infusion. Viral control was associated with an increase in CD8+ effector T-cells (but not CD4+ cells) at a median of 59 days post-3rd party T-cell infusion (Figure 1). Recovery of CMV pp65-specific immunity by ELISpot analysis was observed at a median of 44 days post infusion (pre 4 (0-65) v post 49 (0-309) SFC/105 cells, p<0.0001). Despite the excellent viral control in the presence of recovery of lymphocyte number and function, persistence and expansion of 3rd party T-cells could not be identified. Chimerism testing using droplet digital PCR showed that 3rd party VST DNA was present only at extremely low levels in blood and did not persist beyond 27 days post infusion (Figure 2). TCR sequencing identified clones from the 3rd party T cell product after infusion in only 1 of 3 cases studied, and in that case for only 24 hours after infusion. Instead, the lymphocytes recovering after 3rd party infusion comprised T cell clones that had been present at very low frequency prior to 3rd party T-cell infusion and that were derived from the HSCT transplant donor. We conclude that 3rdparty VST provide prolonged anti-viral benefit mediated via a mechanism that facilitates recovery of HSCT-donor derived virus-specific T-cells.
Figure 1. Recovery of immune subsets in peripheral blood after infusion of 3rd party virus-specific T-cells
Figure 2. Presence of 3rd party virus-specific T cell DNA in peripheral blood after infusion of 3rd party virus-specific T-cells
Yong: Novartis: Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding; Celgene: Research Funding. Gottlieb: Abbvie: Membership on an entity's Board of Directors or advisory committees; Indee: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal